
There is no treatment or prophylaxis against ASF, so rapid diagnosis and strict health and control measures are the basis for the fight against this disease.
It is in the area of diagnosis where most progress has been made, with the development of highly sensitive specific techniques that provide reliable diagnosis of acute clinical, chronic or inapparent forms in a few hours.
There are many techniques for detecting the virus and its specific antibodies. Many of them have been successfully used in control and eradication programmes. The ASF diagnostic techniques most frequently used nowadays are: PCR (conventional and real-time) for virus detection and ELISA and Immunoblotting for serological analysis The following figure on the right represents the most common techniques used in the references laboratories and its application in function of the sample:
For ASF analysis spleen, kidney and lymphatic ganglion tissue samples must be sent, also blood taken when the animals have high temperatures, with an anticoagulant (EDTA). Serum from suspect animals is used for serological tests.
A clinical record sheet must be attached to the exterior of the sample packaging in a sealed envelope.
The following information must be included:
Many ASFV detection techniques have been developed. Some, such as the sandwich ELISA and Immunoblot are not normally used in control and eradication programmes because, although they are highly sensitive in the early stages of infection, this sensitivity decreases drastically from 9-10 dpi.
The most commonly used techniques for detecting the virus are Haemadsorption, Direct Immunofluoresence and PCR.
The ASF virus is isolated in primary cultures of porcine macrophages. ASFV can infect and replicate naturally in porcine peripheral blood leucocytes, where besides producing a cytopathic effect in the infected macrophages, prior to cell lysis it causes a characteristic effect of Haemadsorption (HAD). The microscopic image shows a morula or crown (rosette) of erythrocytes around the leukocytes.
Some ASFV strains are not haemadsorbent. In these cases, additional analyses should be carried out on the cell sediment, by PCR or DIF to confirm the presence of the virus.
Haemasdsorption is still the most sensitive and specific method for identifying ASFV because the other porcine viruses do not produce this effect. It is the reference technique in spite of being laborious and not as rapid as other methods because it takes 5-10 days to obtain results. It is normally used only is reference laboratories.
DIF is recommended when no PCR is available. This technique should be used together with other serological tests (ELISA or IB) to avoid false negatives results caused by animal antibodies blocking.
DIF is based on the detection of viral antigens in tissue cuts or imprints by a reaction with an antivirus fluorescent conjugate. It is a very simple rapid and sensitive method that can also be used on culture cells infected with macerated organs or tissues from suspect pigs. The microscopic images show the presence of intense fluoresence cytoplasmic inclusions in the infected cells. When the infection is advanced, specific fluorescence may have a granular appearance.
In areas where ASF is enzootic, where subacute and chronic forms of the disease are predominant, the sensitivity of this technique diminishes and is only 40% when used directly on organ cuts or imprints.
The combined use of DIF and Indirect Immunofluoresence (IIF) or ELISA enables the detection of 85 to 95% of all cases (even in presence of antibodies) in less than 1 hour and 15 minutes. There are no commercial kits available for it.
PCR (Polymerase Chain reaction) is the technique most frequently used for viral detection. It needs good training and good laboratory practices to avoid contaminations and false positive results. It is a very sensitive specific technique that reveals the presence of the virus by amplifying viral DNA in the sample. It is currently used in reference laboratories for virological diagnosis and confirmation of this disease. It is used in both tissue and serum samples from animals with clinical signs, as it produces prolonged viraemia. The virus is detected in the blood from the second day of infection for weeks.
This technique uses primers directed towards a highly conserved area of the genome, enabling detection of the wide range of known ASFV isolates, including haemadsorbent and non-haemadsorbent strains.
This technique is particularly useful for detecting the virus in badly conserved tissues. PCR results could be obtained in 5-6 hours.
There are two different types of PCR for ASF diagnostic. In both of them is necessary to macerate the tissues and extract the DNA before the PCR method (watch video):
Conventional PCR (watch video) (protocol): products obtained after the amplification of the viral DNA, will be shown by electrophoresis in agarose gel and the use of Ultraviolet light.
Real-time PCR (watch video) (protocol): For this technique it is necessary to use special equipment for the lecture of the fluorescence. Real-time PCR presents some advantages like the simultaneous reading of the results, the possible quantification of the amplified DNA and there is no need to make the electrophoresis.
Further sequencing of PCR products allow us to give positive results with higher confidence, as well as to genotype the virus strains by the amplification of some genome regions (p72-gene, p54-gene, Central Variable Region-CVR).
As there is no vaccine against ASFV, the presence of specific antibodies against the virus is indicative of the presence of the disease.
A wide range of different techniques for serological diagnosis, even directly under field conditions have been developed and used. At present, those most commonly used for detecting ASF antibodies are Indirect Immunofluoresence (IIF), indirect ELISA and Immunoblotting (IB).
Serological techniques are the basis of laboratory diagnosis in ASF control and eradication programmes thanks to their high level of sensitivity and specificity.
This is a rapid technique with good sensitivity and specificity, by which specific antibodies in serum or exudates are made to react on a cell coating infected with the virus. It is not used at present because there are no commercial kits available for it. It takes approximately 2 hours.
In the case of positive samples on the cell tapetum, fluoresence is located at determined sites close to the nucleus, which are the centres of virus replication.
This method is used for carrying out epizootic studies and large scale control studies. It is highly sensitive and specific, simple, rapid and economic. It also has good reproducibility and the results are easy to interpret.
New ELISAs with non-infectious reagents have recently been developed, which use p32, p54 and pp62 recombinant proteins as viral antigens. They have greater sensitivity and specificity in the analysis of badly conserved sera.
There are two different ELISAS for the detection of anti-ASF antibodies:
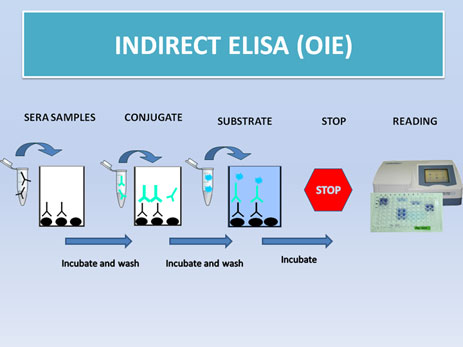
-Indirect ELISA-OIE; this home-made ELISA was developed in CISA-INIA, and it is approved as an official diagnostic technique by the OIE: (watch video) (protocol)
The current ELISA technique uses a soluble antigen containing most of the virus proteins.
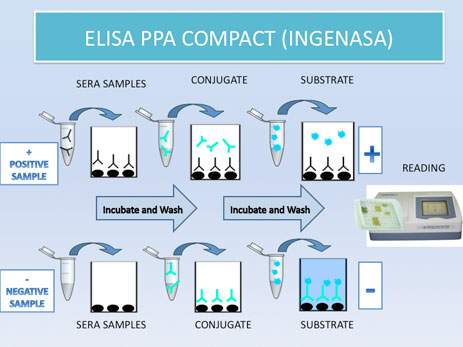
- Commercial ELISA (INGENASA): (watch video) (protocol)
This is a blocking ELISA for the detection of specific antibodies anti vp73 of ASFV (structural protein with high antigenic power)
In this case, ELISA plates are covered with vp73. After the sera addition, a monoclonal antibody anti-vp73 will be added, marked with peroxidase to cover the free plates, where no antibodies have done the antigen-antibody fixation. The interpretation of the results is opposite as the previous ELISA. More color means less level of antibodies in sera samples, and vice versa.
This is an immunoenzymatic technique that uses nitrocellulose filters as the antigenic support, with previously-transferred viral proteins on which the suspect serum is made to react. The specific antibodies are detected with A peroxidase protein.
The IB technique enables the reactivity of the antibodies in the serum to be determined against different proteins specifically induced by the ASF virus. This, together with its high level of sensitivity, and objectivity, make the technique ideal for serological diagnosis when confirming African Swine Fever.
It is highly sensitive, easy to perform and does not require any special equipment, but there are no commercial kits available for it. It takes approximately 3 hours.
Another great advantage of this technique is that the antigen strips can be stored at room temperature, without losing activity for over one year, which means they can be safely transported.