
The clinical signs associated with ASF are very varied as they depend on how virulent the viral isolate is and on the breed and physical condition of the pig. African ASFV isolates generally induce peracute or acute disease. European domestic pigs and boars are very susceptible and exhibit a wide range of clinical signs from subacute to chronic. Wild African pigs are very resistant to infection and do not generally present any lesions.
In the acute form, which is caused by highly virulent isolates (death 7 dpi or before), the animals show high temperatures (40-42ºC) (Fig.1), recumbency (Fig. 2) and lack of appetite, huddle together and in the final stages, suffer respiratory disorders characterised by rapid laboured breathing, with serous or seromucous nasal secretions (Fig. 3) caused by pulmonary oedema setting in.

Fig.2: Pig with a high temperature,
its head is bent forward and cyanosis
of the ears can be observed.
Experimental ASF.
In some cases there may be nasal haemorrhaging, constipation and vomiting, and to a lesser extent, diarrhoea. Haemorrhagic discharge from the anus (melena) is sometimes observed. Exanthemas are very evident (pinkish almost purple skin due to intense hyperaemia), and/or cyanotic foci, which appear as irregular purple-coloured marks on the skin of the extremities (Fig. 4.1 and 4.2), ears (Fig. 5), chest, abdomen (Fig. 6) and perineum (Fig. 7), and also haematomas (Fig. 8) and necrotic areas, though these lesions are more intense in pigs inoculated with moderately virulent isolates. Abortion frequently occurs in gestating females. In acute cases, the disease causes death in 90 to 100% of affected animals.
Animals killed by highly virulent isolates (in peracute and acute courses of the disease), have severe pulmonary oedema and splenomegaly (Fig. 9). The spleen becomes purplish, almost black in colour, crossing the entire abdominal cavity from one side to the other when opened (Fig. 10). This lesion, which is very characteristic of ASF has been called hyperaemic splenomegaly, haemorrhagic infarction and haemorrhagic splenitis (Fig.11). They also show haemorrhaging in the lymphatic ganglia (Fig.12), particularly in the gastrohepatic (Fig. 13) and renal (Fig. 14) ganglia, which almost always affect the cortical and medullar layers, and petechial haemorrhaging in the kidneys, bladder mucosa (Fig. 15), pharynx and larynx (Fig. 16), pleura and heart, (endocardium (Fig. 17) and pericardium (Fig. 18), hydropericardium (Fig. 19)), ascitis (Fig. 20), and hydrothorax and hepatic congestion.

Fig. 9: Spleen. Splenic infarction.
Splenomegaly, congestion, blackish
discolouration androunded edges.
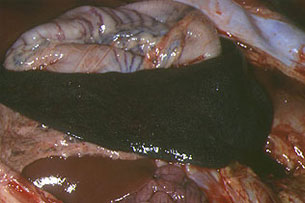
Fig. 10: Spleen. In situ image of the
hypertrophied organ, crossing the entire
surface of the abdomen.
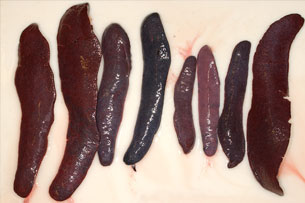
Fig.11: group of spleens with different
levels of hyperaemic splenomegaly
and haemorrhagic infarction
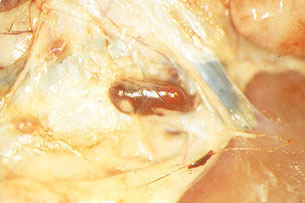
Fig. 14: Renal ganglion with partial
haemorrhaging, kidney with normal appearance,
in acute ASF caused by a highly virulent
isolate.

Fig. 20: Abdominal cavity. Slightly turbid,
yellowishserous liquid, with pale adjacent
intestinal loops.
With moderately virulent isolates the clinical signs vary depending on the course of the disease. In acute and subacute courses death occurs between 7 and 20 dpi, the signs develop more slowly and the disease produces temporary thrombocytopenia causing death in animals that do not survive it.
In animals killed by moderately virulent isolates, the vascular lesions are much more marked, especially in the gastrohepatic (Fig. 21) and renal (Fig. 22) ganglia, kidneys (Fig. 23, 24, 25 and 26) and gall bladder (Fig. 27 and 28), than in acute courses as the lesions develop fully. Also, pulmonary oedema does not usually occur, though pulmonary haemorrhaging does (Fig. 29 and 30). The lesions in the spleen are not very marked and sometimes only affect part of it (Fig. 31), so if the animal does not die it can be cured by cicatrization. Serous to fibrinous pericarditis is also observed, as well as foci of necrotic pneumonia. Haemorrhaging can also occur elsewhere, for example in the submandibular and retropharyngeal lymph (Fig. 32), mediastinal (Fig. 33), inguinal (Fig. 34) and mesenteric (Fig. 35) ganglia, serous layer of the small and large intestine (Fig. 36), fundic mucosa of the stomach (Fig. 37) and skeletal muscle (Fig. 38).
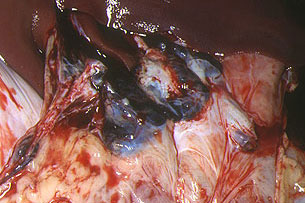
Fig. 21: Gastrohepatic ganglia. Completely
haemorrhagic appearance, with hypertrophy,
and blackish in colour. Experimental ASF
induced by a moderately virulent isolate.
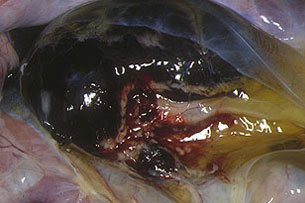
Fig. 22: Haemorrhagic renal ganglion, similar to
a blood clot. In situ image showing
the dark-coloured kidney and yellowish
jelly-like substance produced by perineal oedema.
Experimental ASF induced by
a moderately virulent isolate.
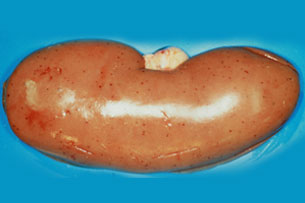
Fig. 23: Kidney. Petechiae covering the
entire surface of the renal cortex.
The colour of the renal parenchyma
is normal. Experimental ASF induced
by a moderately virulent isolate.
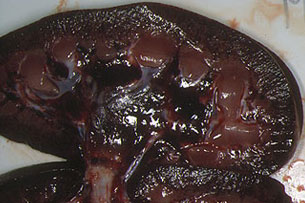
Fig. 24: Kidney. Axially cut, revealing
haemorrhaging in the cortical and medullar layers
and renal pelvis. Experimental ASF induced
by a moderately virulent isolate.
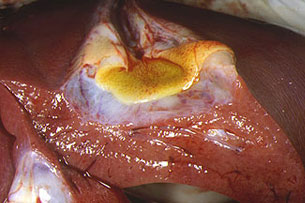
Fig. 28: Transversal cut of the gallbladder.
Oedema in the gallbladder serose, separated
from the liver by a whitish gelatinous band.
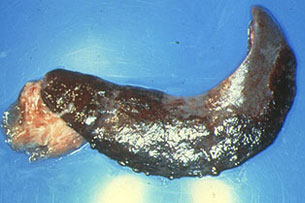
Fig. 31: Spleen. Infarction affecting
75% of the organ. The affected area is separated
from the area of cicatrization.
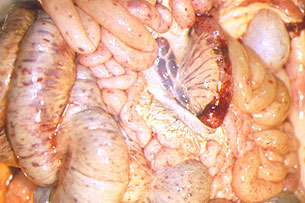
Fig. 36: Intestine and mesenteric ganglia.
Petechial haemorrhaging and subserose
equimosis in the small intestine, colon
and caecum, and haemorrhaging
in the mesenteric ganglia.
The chronic form is generally caused by isolates of low virulence, or is detected in resistant populations. It is characterised by a large variety of clinical signs which are mainly the result of secondary bacterial complications, the most significant being reproduction and articular alterations. Mortality is low, affecting between 2 and 10% of all the sick animals. Abortion usually occurs, intense necrotic processes affect the skin and buccal cavity, as well as arthritis, causing lamenes.
The most characteristic microscopic lesions in ASF are haemorrhaging, particularly in the renal and gastrohepatic lymph ganglia and kidneys. These lesions, as indicated in the "Pathogeny" section, are the result of phagocytic activation of the endothelial cells and loss of the capillary endothelium in acute ASF, and also due to increased vascular permeability (which causes interstitial oedema) and erythrodiapedesis in ASF. Apoptosis of the lymphocytes, in both infiltrates and lymphoid structures, could be the cause of lymphopenia, though the vascular lesions cause the inhibition of lymphocyte proliferation and destruction of the lymphoid structures of the spleen, ganglia and tonsils at the same time.