
The aetiological agent of ASF is a large enveloped icosahedral DNA virus. Its characteristics and morphology are similar to those of Iridoviruses, and the organisation of the genome is similar to that of the Poxviruses. It has recently been reclassified in a new family Asfarviridae, genus Asfivirus of which it is the only member.
The viral particle has a diameter of 200 nm and is composed of three concentric envelopes, with another external hexagonal envelope which forms due to gemmation through the membrane of the infected cell.
The hexagonal icosahedral capsid surrounds an electrodense nucleus with a diameter of 80 nm, which in turn, is surrounded by a lipidic envelope.
| Characteristics of the viral particle |
| Mean size of approximately 200 nm |
| Electrodense nucleus of 80 nm |
| At least three envelopes, and another one in the extracellular virus |
| Characteristics of the viral genome: |
| Molar mass: 100.1 gr mol-1 |
| Density in ClCs: 1.7 gr. Cm3 |
| Sedimentation coefficient: 60s |
| Length: 58nm |
The viral particles are thermolabile and sensitive to lipidic solvents, but very resistant to a wide range of pH.
Replication occurs in the cell cytoplasm, although DNA virus synthesis takes place in the nucleus. The ASFV has its own transcriptase enzyme, a DNA-dependent RNA polymerase.
The 170-193 kb double strand viral DNA is inside the viral particle, depending on the viral isolate, similar to the vaccine virus (virus vaccinia), with the ends covalently bound and terminal-inverted repeats (TIR).
Variability studies show that viral DNA is made up of a more preserved central area (with a region of certain variability) of approximately 125 kb and two variable areas at the ends of the molecule, where diversity between the isolates is greatest. Different ASFV isolates have been classified in groups based on the conservation of the central region of the genome.
| Some physico-chemical characteristics of the ASFV |
| It is inactivated at pH<4 and pH >11, though depending on the medium used, it can take hours or days for inactivation to occur. |
| It is inactivated at 56ºC for 60-70 min. and 60ºC for 20 min. |
| Inactivants/Disinfectants: |
| 2% sodium hydroxide for 30 min. |
| Hypochlorite (2-3% chlorine) for 30 min. |
| Formalin (3/1000) for 30 min. |
| Phenolic compounds |
| Iodide compounds |
The viral genome of isolate Ba71 has been completely sequenced. The DNA is composed of 170,701 nucleotides and contains 151 open reading frames (ORFs), and 5 multigene families (MGFs).
The MGFs are adjacent to the TIRs inside the genome, in the terminal variable regions. There are at least 5 MGFs (MGF360, MGF530, MGF10, MGF300, MGF505), and this is where the greatest differences between the ASFV isolates are found, which could indicate that the regions are related to the generation of antigenic variability in the virus, a possible evasion mechanism of the virus from the immune system.
| ASFV genotypes |
| ASV does not induce neutralising antibodies and this is the reason of non serotype classification. |
| Discrimination between isolates is achieved by genotyping strategy based on the partial p72 nucleotide sequence (B646L gene), differentiating up to 22 genotypes so far identified. |
| Subtyping is carried out by full genome sequence of the p54-gene. Enhanced discrimination between the 22 genotypes can be obtained by analysis of the central variable region (CVR) within B602L gene. |
| In Africa, a total of 22 genotypes have been identified to date. The genotype I isolates are mainly present in west African regions. The other 21 genotypes have been detected in eastern and southern areas. |
| The old outbreaks occurring in Europe and central and south America, genotype I was the only affecting. However, since 2007, a new genotype has been emerge in Europe, in the Caucasus region, characterized as genotype II, similar to those circulating in eastern African countries. |
As many as 34 structural proteins and over 100 infection proteins have been identified in macrophages infected by the virus. At least 50 of which are immunogenic and induce antibodies in natural infection. Some of these proteins, for example the p72, p54 (25 kda) and p12 structural proteins, involved in the binding of the virus to the cell, are highly antigenic, or p32, involved in events related to virus internalisation. Although these proteins have not been related to the induction of immune mechanisms that confer total protection during infection, they are very useful as antigens in serological diagnosis.
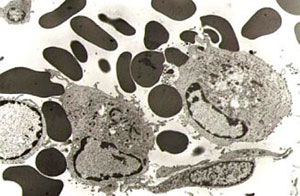
Cells in the monocyte-macrophage system
infected with ASFV, where the
haemadsorption phenomenoncan be observed.
The virus naturally infects monocyte-macrophage cells in the immune system, producing characteristic haemadsorption before the infected cell is destroyed. Viral replication has also been observed in endothelial cells, hepatocytes, renal tubular epithelial cells and neutrophils. Infection has not been described in T or B lymphocytes.
It also replicates naturally in O. Moubata and O. Erraticus ticks.
The ASFV has adapted to grow and multiply on different stable cell lines where it has a cytopathic effect. Amongst them are the MS (monkey stable) kidney and Vero lines, which are used in research and in producing reagents for diagnosis.
The virus enters the cell via an endocytosis mechanism mediated by a receptor.
The role played by the different humoral and cell based immunological mechanisms during ASFV infection is not yet clear. Different effector cell and humoral mechanisms have been described during ASFV infection, but nothing is known about their role in protection against infection.
ASFV infection produces an enormous specific antibody response, which is easily detected by laboratory diagnosis techniques from day seven post-infection. Specific antibodies remain for a long time, up to years, and are partly responsible for delaying the appearance of clinical signs and reducing viraemia levels. However, these antibodies are not capable of completely neutralising ASFV.