| Chapter 5. How are immunoglobulins studied? | | | | | | | How are immunoglobulins studied? | Porcine immunoglobulins are present in two different forms: as part of the BcR receptor of the B lymphocytes or as antibodies in the serum, body fluids and tissues. Their study represents a key tool in animal health.
| | | The genetic control of immunoglobulin production solves with elegance and creativity what could be a serious problem. | | 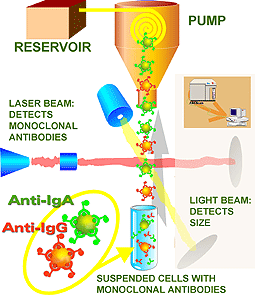 Diagram of flow cytometry for the detection of surface immunoglobulins in B lymphocytes. The cells in suspension, to which have been added the various MAbs (anti IgA and IgG), pass through a very fine tube in a single stream of cells which passes through a laser and a light beam; the former identifies the labelled antibodies and the latter the size of the cells. Diagram of flow cytometry for the detection of surface immunoglobulins in B lymphocytes. The cells in suspension, to which have been added the various MAbs (anti IgA and IgG), pass through a very fine tube in a single stream of cells which passes through a laser and a light beam; the former identifies the labelled antibodies and the latter the size of the cells. | SURFACE IMMUNOGLOBULINS As we saw in chapter 2 How are B lymphocytes studied?, there are several methods used to study the immunoglobulins in the membrane of the B lymphocytes which make up the BcR receptor. The two most frequently used methods are:
| | 
Image of agglutination in tube
| Free immunoglobulins in serum, colostrum, milk, fluids, etc can be studied as total immunoglobulins, without considering which antigen they react against, or as specific antibodies against the different antigens of interest. A large number of techniques are available for the latter type of study, which is undoubtedly the most interesting. These range from the classic methods of precipitation, complement fixing, etc, to the most modern and increasingly used immunoenzymatic techniques. | |
| Sensitivity of the various techniques for the detection of antibodies (mg/ml) | | METHOD | SENSITIVITY (mg/ml) | | Gel precipitation | 30 | | Ring precipitation | 18 | | Bacterial agglutination | 0.05 | | Complement fixing | 0.05 | | Passive hemagglutination | 0.01 | | Hemagglutination inhibition | 0.005 | | Immunofluorescence | 0.005 |
| ELISA | 0.0005 | Bacterial neutralization | 0.00005 |
| Differences in sensitivity, specificity, capacity for use on a large scale, etc are some of the most important concepts when choosing which technique to use. Using sensitivity as an example we can see that there are low level techniques such as precipitation, techniques with average sensitivity such as bacterial agglutination and complement fixing, and others with greater sensitivity such as ELISA, serum neutralization or bactericide activity.
The development of molecular biology and the production of monoclonal antibodies means that over recent years highly sensitive and specific diagnosis reagents have become available, even in the form of kits which are easy to use and interpret.
Amongst all the methods currently available, in this chapter we highlight those which offer the greatest possibilities for large-scale serological studies which can be carried out without the need for many technical resources. We will also review other methods which allow a lower number of samples to be studied, but which offer good sensitivity and specificity and do not require a lot of equipment. Finally, we will look at a reference technique for all serological studies, which although requiring greater infrastructure and longer performance time, has been included due to its importance.
| | The main methods with these characteristics are:
| |  | - IMMUNOELECTROTRANSFERENCE OR WESTERN BLOT
| | - INDIRECT IMMUNOFLUORESCENCE OR IMMUNOPEROXIDASE
| | | | | | | ELISA The immunoenzymatic technique ELISA is a serological method which uses conjugates to visualize the ANTIGEN-ANTIBODY reaction. ELISA is based on the use of antibodies labelled with an enzyme (usually peroxidase) so that the resulting conjugates have both immunological and enzymatic activity. When one of the insolubilized components (antigen or antibody) is on the plate, the antigen-antibody reaction remains immobilized and can thus be easily revealed by the addition of the substrate. When this acts on the enzyme, it produces a colour which is visible to the naked eye or quantifiable by a colorimeter. There are several commercial kits available and therefore serological studies can be carried out without the need for many resources. | 
On the first line the positive control serum can be observed and on the second the negative. The serum has been placed on the rest of the plate in duplicate; positive (blue) and negative (colourless) are observed. |
ELISA is currently one of the most commonly used methods for the serological diagnosis of different infectious diseases in swine.
The ELISA method is mainly recommended for the study of populations. It is a highly sensitive and very specific technique which enables studies of large populations to be carried out easily and economically in a short space of time. This technique also offers good reproducibility and ease of interpretation of the results.
Several variants of the ELISA method have been adapted for the determination of both ANTIGENS and ANTIBODIES:

| 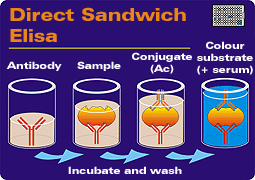
Phases of the Sandwich Elisa technique. (1) A monoclonal or polyclonal anti-antigen antibody is already attached to the plate. (2) This is incubated with the sample. (3) The conjugate is added. (4) Finally the substrate is added. All steps are preceded by incubation and washing. | ANTIGEN DETECTION The most common version of the ELISA method for antigen detection is the "Sandwich" ELISA model. In this version, the plate usually contains a fixed antibody (monoclonal or polyclonal) against the unknown antigen. A macerate of the organ to be tested is added. If this reacts with the antibody on the plate, it will become evident after the addition of the second antibody labelled with the enzyme. Finally, the substrate is added to reveal the reaction.

|
ANTIBODY DETECTION
The following versions of the ELISA method are usually used to determine specific antibodies against a particular antigen: -
INDIRECT ELISA. - COMPETITIVE ELISA.

| | 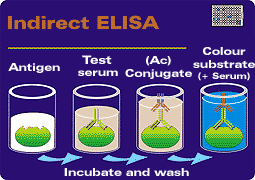
Phases of the indirect Elisa technique. (1) The antigen is bound to the plate. (2) The serum is added, (3) then the conjugate, (4) and finally the substrate is added.
|
INDIRECT ELISA. This is the most commonly used method for antibody detection. In brief, it consists of coating the ELISA plate with the antigen (this has already been done in kits) under investigation. The test reveals whether there are specific antibodies present in the serum. It can be used with antigens, viral or bacterial proteins and even whole viruses.
It is increasingly common to use only those proteins of immunological interest and not all antigenic proteins. The next steps are the addition of the serum, incubation and washing, addition of the conjugate, incubation and washing, finishing the process with the addition of the substrate, stopping the reaction and reading the results.  | | 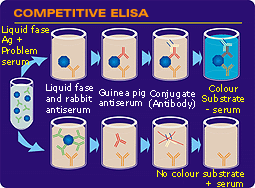
Phases of the competitive Elisa technique. (1) The serum is incubated with the antigen. (2) This blend is placed on the wells where an anti-antigen serum has previously been fixed. (3) The conjugate is added, (4) then the substrate. In this case the absence of colour means a positive sample. |
COMPETITIVE ELISA This system is also often used for the detection of specific antibodies. It is based on an antibody (monoclonal or polyclonal) against a known antigen which has been previously placed on the plate. The method is called competitive as the serum is previously incubated with the antigen before incubation with the antiserum fixed on the plate, thus competing with it. The following steps are the addition and incubation of the conjugate, washing and finishing with the substrate and reading the result.  | | 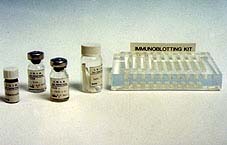
Material necessary for immunotransfer: Nitrocellulose sheets with antigens, PBS buffer, positive and negative reference serum, conjugate, substrate solution and plastic support to carry out all processes.
| IMMUNOELECTROTRANSFERENCE OR WESTERN BLOT Immunoelectrotransference, "western blot" or "Immunoblotting" is an immunoenzymatic technique used for the detection of specific antibodies. It is recommended when it is not necessary to study a large amount of serum or to analyse serum which has given doubtful results using other techniques. This method uses antigenic nitrocellulose filters as a substrate, to which the proteins of the antigen have been previously transferred, totally or partially maintaining their antigenic properties. In order to obtain the antigen filters, the proteins are first separated by polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred by electrical current to the nitrocellulose filter. This filter is cut into small strips, which will become the antigen substrate. The serum and the positive and negative serum control are incubated on each of these strips. After a period of incubation and subsequent washing the conjugate is added to anti-immunoglobulin G or M (depending on which is to be studied). If there are immunoglobulins bound to the antigenic proteins they will be revealed by the conjugate. One or several lines of specific precipitation can be observed depending on the presence of antibodies against one or several proteins. Specificity is shown when there is a reaction with proteins of the same molecular weight as antigenic proteins.
| 
Final phase of the technique. The appearance of the different lines where the test and control serum have reacted can be observed.
|
This is a very sensitive technique which is easy to handle, carry out and interpret. No special equipment is needed. This technique is mainly indicated in the study of smaller amounts of serum. As it does not require special equipment it is easy to carry out even in laboratories with limited equipment. These days several kits are available to test for different diseases, with an increasingly large choice available.  | | 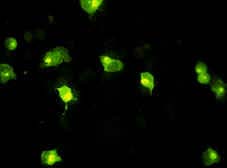
Indirect immunofluorescence technique. MS cells infected with the African swine fever virus. It can be seen how the antibodies have bound to the infected cells. Certain areas with greater intensity can be noted in the cytoplasm, corresponding to areas of higher viral replication, and thus greater fixing of antibodies. | INDIRECT IMMUNOFLUORESCENCE OR IMMUNOPEROXIDASE Indirect immunofluorescence or immunoperoxidase, depending on whether fluorescein isocyanate or peroxidase is used, are techniques which use the specificity of histology (the cell can be seen and it can be proved that the immunological reaction takes place in a specific site) alongside the sensitivity of immunological techniques.
These techniques normally use cell cultures (primary or stable) infected with the virus or the bacteria which we wish to test to find out whether there are antibodies in the unknown serum. After incubation with the serum, if there are antibodies they will bind to the infected cells but not to the non-infected cells (observable histological specificity). The binding can be seen after the addition of an anti-immunoglobulin conjugate labelled with isocyanate or with peroxidase and subsequent observation with fluorescence or conventional microscope respectively.

| | 
Infected cell layer.
Phases of the competitive Elisa technique. (1) The serum is incubated with the antigen. (2) This blend is placed on the wells where an anti-antigen serum has previously been fixed. (3) The conjugate is added, (4) then the substrate. In this case the absence of colour means a positive sample.
|
SERUM NEUTRALIZATION This method is considered as the reference point for any serological study as it is that which most correlates between the "in vitro" response and the "in vivo" response. This test evaluates the capacity of the antibodies present in a serum to neutralize the biological activity of an antigen. The methods described up to this point allow the capacity of a particular serum to recognise a specific antigen to be assessed; serum neutralization takes another step forward and indicates the capacity of a serum to neutralize the biological activity of the antigen. These methods are widely used in laboratories to assess the capacity of a serum against bacterial toxins, bacteria and/or viruses. These tests are more laborious, requiring cell cultures and sterility, as well as being on the whole slower than the former. However they are highly specific and sensitive, and are considered as the point of reference for any serological assessment. In the case of viruses, the capacity of a particular serum to neutralize the infectivity of the virus on a sensitive cell lineage can be measured. A viral solution, at a constant concentration and which has previously been in contact with different dilutions of the serum, is added to the cells. The cells to which the various dilutions have been added are observed to see whether the virus has infected them or not; this is by means of staining with a conjugate or by cytopathic effect. In this way the capacity of viral neutralization of an unknown serum can be measured.  | | | |

