| |
Serum profiles are based on the detection of circulating antibodies using any of the techniques currently available, with the aim of obtaining information on the condition of the animals in a piggery at a given time, or during previous stages. These studies are becoming increasingly used for the health control of piggeries, in many cases representing a means of significantly reducing costs of drugs and increasing the level of health.
Serological analysis allows us to, amongst other possibilities: find out the health condition, choose the best time for vaccination, control vaccination programmes and prevent disease from entering the piggery. |
|
In order to carry out a successful serological study in a piggery the following points should firstly be taken into account:
- What do I wish to find out about my piggery? What questions do I want to ask? Can serology give me an answer to these doubts?
|
 |
- About which animals should we ask these questions and when?
|
 |
- How many samples should I take and how can I do this?
|
 |
- What differences are there between laboratory techniques?
|
 |
|
|
1. What do I wish to find out about my piggery?
This is the first question to be clarified before starting a study. ¿What do we want to know about our farm? As we will see later, serology can help us find out certain things but not others. It is important to know these limitations. The dynamic of appearance of antibodies, the difficulty in differentiating between vaccination antibodies and disease antibodies, the time taken by antibodies to appear after an infection, etc are some of the considerations to be taken account when planning a serological study.
I want to know if there are any diseases present...How is the vaccination programme working? What is the health status of replacement animals?, etc. These are some of the questions that should be asked and for which serological studies are indicated. In applications of serology we will see how these studies can be set up.

|
|
2. About which animals should we ask these questions and when?
This is another important question that should be clearly defined. Remember that animals at different production phases can present different serum profiles; we should ask this question about the animals which can provide the answer. For example, it must be remembered that immunoglobulins do not cross the placenta, meaning that a piglet does not have immunoglobulins present until it drinks the maternal colostrum.
The animal starts to acquire humoral immunity when the colostrum is drunk. This humoral response is the reflection of the mother’s immunity and is still not that of the piglet itself. The maximum adsorption of the colostrum takes place between 6 and 36 hours after birth, with the presence of immunoglobulins in blood detected between 12 and 24 hours after ingesting the colostrum, staying in the bloodstream for times which vary depending on the type of immunoglobulin, immunological status of the mother, type of infectious agent, etc. In general, colostral immunoglobulins are present between the 3rd, 8th and 20th weeks of life of the animal. Depending on the question being asked, for this and other reasons it is important to know at what age to take the sample and from which animals: Mothers, piglets, fatteners, etc.

|
|
3. How many samples are necessary for a serological study?
This is another of the key questions to ensure that the results are representative of the situation. As well as taking samples from the different areas of the piggery and the different production areas it is also important to define how many samples are needed from each area. Two aspects are important when evaluating a piggery: Those which aim to determine whether a disease is present in the piggery and, if it is present, studies which intend to discover its prevalence. In other words, the questions would be: Is this disease present in my piggery? If the answer is “yes”: How prevalent is it?To answer this question it is vital to determine the size of the sample that should be analyzed. This sample size will also depend on the degree of precision we need to obtain. Tables are available for these studies (see other sources of information) which indicate the number of samples needed in relation to the expected prevalence, precision and level of confidence required. The number of samples needed varies a great deal depending on the level of confidence we wish to obtain and the suspected prevalence. From a practical and simple point of view, the following figures can generally be applied to serological sampling:
For breeding sows:
-
In piggeries with fewer than 25 animals: All are studied
-
In piggeries with between 25 and 100 breeding sows: 25 samples are taken which are rotated in subsequent samplings.
-
In piggeries with more than 100 breeding sows: 30 samples which are rotated.
In fatteners:
The other question would be: What type of sample do I need and how do I take it? The serum profiles are carried out mainly on the serum of the animals, although at times it is also of interest to find out the amount of immunoglobulins present in the colostrum and/or in maternal milk. The colostrum samples should be collected during the 24 hours after birth. If they are to be sent immediately to the laboratory, they should be refrigerated. If they are sent during the following days they should be frozen.
|
|
Blood used to obtain serum is usually extracted from:
Marginal vein in the ear. Either using a lancet and collecting the blood in a tube, or directly using a syringe and 16 mm to 25 mm needle. The advantage of this system is its simplicity, but the main disadvantage is the small amount of blood that can usually be obtained and the high risk of contamination.
Tail vein. Syringes (25 mm needles), vacutainers or partial amputation are used. It is a relatively simple method but a sample of only around 0.5 ml can be obtained. This is not the ideal method.
Jugular vein. This is one of the most commonly used methods, particularly in mothers. Syringes, "vacutainer" or "monovet" are used with needles appropriate to the size of the animal. Between 10 and 30 ml can be extracted.
Vena cava. Used to extract blood from all types of animal from piglets to adults, above all when a large amount of blood is needed. The needle size varies according to the animal's weight (10 Kg. 25 mm, 45 Kg. 38 mm, 100 Kg. 50 mm, mothers 100 mm).
Ocular bleeding. Used to extract blood from all types of animal from piglets to adults, above all when a large amount of blood is needed.
Finally, What do I do with the extracted blood? The blood should be kept at room temperature (in a cool area out of sunlight) until it coagulates (approximately one to two hours). It should then be kept refrigerated at + 4º C overnight. Next, it is recommended that the blood be centrifuged, or the serum at least should be separated from the coagulate before sending to the laboratory. The tubes should be clearly marked and carefully packed. If the study to be carried out involves two determinations with a 21 day interval (seroconversion), it is better to freeze the serum once it has been coagulated and separated from the coagulate. After the second sample has been taken and processed in the same way, both serum samples can be sent at the same time. A successful diagnosis largely depends on a good sample. Avoiding contamination or alterations to the serum is always a guarantee for the diagnosis.
 |
|
4. What are the differences between laboratory techniques? As we saw at the beginning of this chapter, nowadays there are many laboratory techniques available for serological studies. The diagnostic capacity of a method is determined by calculating the sensitivity and specificity of a particular method in comparison to the reference technique. Several concepts should be remembered when assessing the techniques available. These are:
Sensitivity: This is the capacity of a method to detect positive serums against a particular disease. Sensitivity enables all positive cases to be detected.
Specificity: This is the capacity of a method to differentiate positive serums from negative serums in a particular disease. Specificity avoids false positive results. No negative result should be considered as positive by any technique.
Predictive value: This is the capacity of a technique to distinguish between animals suffering from a particular disease and those which are not; in other words, predicting sensitivity and specificity for a negative or positive case. The predictive value can be:
Positive: This is the frequency of the disease amongst animals with a positive result.
Negative: This is the frequency of the absence of the disease amongst animals with a negative result.
Efficacy: Percentage of animals correctly classified by a particular method.
True positive: Sick animal correctly classified by a technique.
False positive: Animal incorrectly classified by a technique.
|
|
The greater the differences obtained in the distribution of positive and negative populations which establish the cut off point, the greater the sensitivity and specificity of a technique. 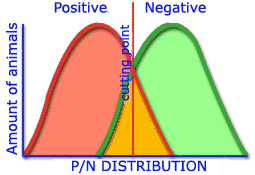
Low sensitivity and specificity
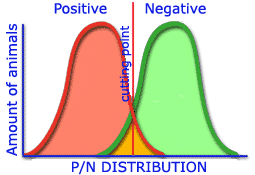
Medium sensitivity and specificity
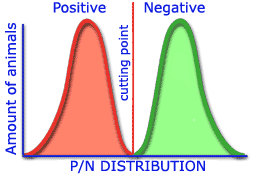
High sensitivity and specificity |
Assessment of the sensitivity, specificity and predictive value parameters of a technique is carried out by comparing the results with a reference technique, or with the disease itself, using the following formula:
|
Control technique or real infection |
|
Technique being assessed
|
|
+ |
- |
| + |
A |
C |
| - |
B |
D |
A Positives in which both techniques coincide.
B Real positives that the technique under assessment gives as negatives.
C Positives of the technique under assessment which are actually negatives.
D Negatives by both techniques.
|
SPECIFICITY = |
D
C+D |
x 100 |
Predictive value for positives:
A / A + C
Predictive value for negatives:
C / B + D |
|
Amongst all techniques currently in use, those with the highest levels of sensitivity and specificity are the techniques of serum neutralization and ELISA. Although it depends on the laboratory, other techniques can be used and similar results obtained. The most important factor here is to remember that to compare results at different times, different stages, etc, serums should also be studied with the same technique and even using the same laboratory. If not, different results could be obtained and confound the diagnosis.

|
|
|

