| Chapter 4. Porcine immunoglobulins. | | | | | | | How many different types of immunoglobulin are known? | Four types of immunoglobulin have been found in pigs: IgM, IgG, IgA and IgE. The possible existence of porcine IgD has still not been definitively proved.
| | | None of these immunoglobulins pass through the placenta.
The transfer of immunoglobulins from mother to foetus takes place through the colostrum. Piglets absorb immunoglobulins through the intestine, and in turn they pass into the serum.
|
|
M immunoglobulin.
This is the first immunoglobulin to be produced after an immune response and the predominant isotype in the primary response.
IgM is the second most abundant immunoglobulin in serum after IgG, representing between 10 and 12% of all porcine immunoglobulins. Its concentration in serum is between 1 and 5 mg/ml, between 0.3 and 0.9 in maternal milk and between 2.5 and 3.2 mg/ml in the colostrum.
Different subclasses of IgM have not been described in pigs, but an allotypic variant has been observed in some animals.
As with all immunoglobulins, it can appear as part of the BcR of B lymphocytes; in this case, as a monomer of 180 kDa. It can also be in the form of an antibody secreted into the body fluids, in which case it appears as a polymer of five monomers of 180 kDa each that represents a molecular weight of 900 kDa and a sedimentation coefficient of 17.8 S.
|
|
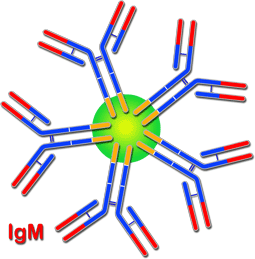
Diagram of the structure of IgM. The J chain is in green
|
The different monomers are bound to one another, creating a pentamer, by a small polypeptide very rich in cysteine, known as the J chain. Each IgM monomer has a structure matching the general description of the immunoglobulins, with two heavy mu chains (m) and two kappa (k) or lambda (l) light chains. IgM does not have a hinge region, although it has a CH4 fragment in its heavy chains where complement activation takes place.
The role of IgM is very important as it is the first defence immunoglobulin in the humoral response. Although its degree of reactivity with the antigen is lower than that of IgG, its pentameric structure allows it to bind with antigens in a multiple manner and so activate the complement. A single pentameric IgM molecule bound to an antigen is able to start complement activation and phagocytosis. IgM is particularly effective against a large number of gram negative bacteria and can neutralize viral agents. Owing to their large size they are mainly found in blood serum.
|
|
Immunoglobulin G.
IgG immunoglobulin is the main isotype in pigs, representing between 80 and 85% of porcine immunoglobulins in serum and colostrum. It is also the most important antibody in the secondary response. Its concentration in serum is between 17- 29 mg/ml, between 1- 3 in maternal milk and between 30 - 70 mg/ml in colostrum. At least five subclasses of IgG have been described: IgG1, IgG2a, IgG2b, IgG3 and IgG4. DNA studies have shown that there are eight genes which code constant region Cg. This immunoglobulin plays a vitally important role in the humoral response. |
|
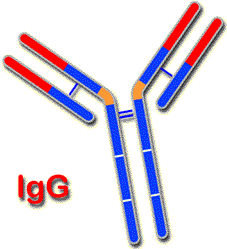
Diagram of the structure of IgG. |
Its structure is the same when it is part of the BcR as when it is in the form of an antibody. In both cases, it forms a monomer of 180 kDa, with two identical gamma heavy chains (g) and two kappa light chains, also identical, (k) or lambda light chains (l). It has a hinge region.
Owing to its small size, IgC can easily leave the bloodstream and enter at tissue level where it has an important defence function. It has a high level of affinity for antigens, and is able to opsonize them to facilitate phagocytosis (chapter 3), agglutinate them or precipitate them. IgG has a significant viral neutralization capability, is vital in antibacterial activity and can activate the complement via two pathways, as well as taking part in ADCC activity.
| Concentration of porcine immunoglobulins |
| mg/ml. |
IgG |
IgM |
IgA |
IgE |
| Serum |
17-29 |
1-5 |
0.5-5 |
- |
| Milk |
1.3 |
0.3-0.9 |
3-7 |
- |
| Colostrum |
30.70 |
2.5-3.2 |
9.5-10 |
- |
|
|

Diagram of monomeric IgA |
Immunoglobulin A.
In pigs, this is the most important immunoglobulin in the immunity of the mucosae and the main immunoglobulin in lactation. Its concentration in serum is between 0.5 and 5 mg/ml, between 3 and 7 mg/ml in maternal milk and between 9.5 and 10 mg/ml in colostrum.
Two subclasses have been described in pig IgA1 and IgA2. |
|
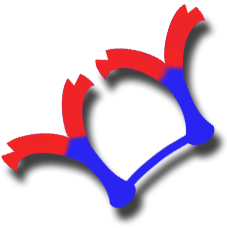
Diagram of IgA in dimeric form bound by J chain |
The structure of basic IgA is that of a monomer of 150 kDa, although it is normally secreted in dimeric form by means of a J chain. It can also be seen in the form of trimers and even major polymers. As in other immunoglobulins, its basic structure is two identical alpha heavy chains (a) and two kappa or lambda light chains. |
|
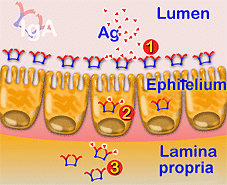
Diagram of the different activity levels of IgA (1) in the lumen, (2) enterocytes and (3) cell fluid
|
IgA activity is essentially related to the immunity of the mucosae where three different levels act preventing the penetration of antigens in the intestinal wall, neutralizing the activity of certain viruses, including within and outside of epithelial cells.
IgA does not activate the complement cascade and neither can it act like opsonin. |
|
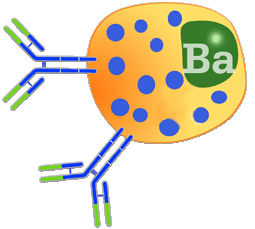
Diagram of the binding of two IgE molecules with the basophil. |
Immunoglobulin E.
IgE represents less than 0.01% of immunoglobulins found in pigs. This immunoglobulin has been determined by functional methods, including being induced by the presence of a virus such as African swine fever.
IgE is a monomer of sedimentation coefficient 8S. As in IgM, its heavy chains have four constant domains (CH). Its molecular weight is 190 KDa which is greater to that of IgG. IgE's Fc fraction has a fragment with great affinity for binding to the basophil membrane where it reacts with the antigen allowing the release of the various inflammatory products contained in the granules of these cells. In pigs, a large amount of histamine is released, above all at intestinal and respiratory level. These defence mechanisms are of key importance in parasitic infections.
|
|
| |

