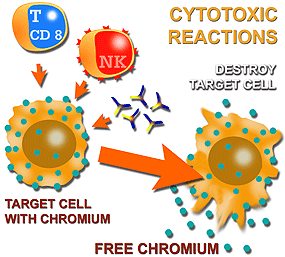
Diagram of cytotoxicity studies and their different forms of induction: by CD 8+ lymphocytes, by NK cells and by complement-activating immunoglobulins. Their evaluation is carried out by measuring chromium 51 liberation in a g radioactive particle counter after destroying the target cell. | CYTOTOXIC ACTIVITY OF T LYMPHOCYTES (CD 8+) The cytotoxic activity of T lymphocytes (CD 8+) against a target cell can be studied by measuring the capacity that a certain number of T lymphocytes have to destroy a certain number of target cells when both populations are in contact. There are several methods to evaluate the percentage of lysis or cell death in the target cells. The most-commonly method, because of its precision, sensitivity and reproducibility, is that of chromium 51 liberation from target cells. In short, this method consists of the following: The target cells (which express certain membrane antigens) are labelled with chromium 51 and put in contact with the effector cells (CD 8, NK, etc) in a suitable proportion. After the incubation period, both cell populations are centrifuged and then a portion of the resulting supernatant is analyzed using a gamma particle counter to find out the percentage of chromium 51 released. The greater the quantity of chromium released, the greater the cytotoxic activity. | 
