| Chapter 9. New generation vaccines. | | | | | | | How many types are known? | One possibility offered by genetic engineering is the elimination of genes which express proteins related to virulence in order to achieve more attenuated strains.
Equally, different genes from different microorganisms can be incorporated into one that acts as a vector. These are the new generation vaccines.
| | |
Thanks to current knowledge of the immune response against infectious agents, and to the development of different genetic engineering techniques, new vaccines are being designed that resolve current problems with the use of conventional vaccines.
|
|
|
| Thanks to the development of molecular biology, advances have been made in our knowledge of the different genes that make up microorganisms and the proteins coded by them. It is therefore been possible to modify the genome structure of some microorganisms, such as the Aujeszky’s disease virus (AD), eliminating genes that code proteins linked to virulence, safely and stably achieving attenuated strains. |
|

Diagram of the structure of the virion, its proteins (with their new nomenclature) and the DNA of the AD virus. 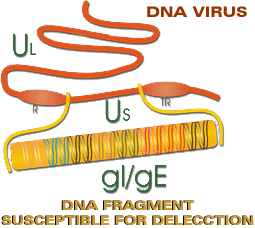
Diagram of the structure of the DNA of Aujeszky’s virus. The genes of gE and gI are located in the Us fragment. |
In the mid-1980s, research into the genome of the AD virus showed that some viral strains used to create live attenuated vaccines presented deletion in the Us region of their genome, affecting the genes which coded gE glycoproteins (formerly known as gI) and gI (formerly gp 63).
The Bartha K/61 strain presented deletion in the Us region which affected gE and gI (formerly gp 63). The NIA-4 strain also presents deletion in the Us region and therefore does not express the gE protein. The Alfort 26 strain presented deletion in the Us fraction but this only affected the expression of gI and not of gE. Therefore, the vaccines produced with these strains induced good protection in the animals, but did not induce antibodies against the gE protein. The animals vaccinated with them could be differentiated from infected animals, as the virus which produces the disease presents the gE protein. 
Diagram of the structure of the AD virus virion with the location of the different proteins.
The discoveries mentioned stimulated molecular research into the AD virus. Using genetic engineering techniques, various studies were set up to eliminate genes which coded or proteins related to virulence (thymidine-kinase) or proteins which, such as gE, are not important in the induction of the immune response and permitted the vaccine strains to be differentiated. Thus, less virulent strains were obtained by eliminating the thymidine-kinase gene and labelled strains by eliminating the gE gene. |
|
| LIVE RECOMBINANTS |
|
Live recombinant vaccines are based on the use of a microorganism (virus or bacteria) which acts as a vector to express genes of a different microorganism. In this manner, this new recombinant microorganism can be used as a vaccine against both. The microorganism which has been used most frequently as an expression vector has been the vaccine virus ("vaccinia"), as it has a wide and well-studied genome which allows foreign genes to be inserted without altering its replication machinery. For example, a recombinant against the rabies virus has been produced by inserting the gene of the G protein of the rabies virus into the genome of the "vaccinia" virus. The disadvantage of this vector is that it is a virus capable of infecting most animal species, including man. A live non-species specific vaccine would be obtained and could therefore immunize any animal. One of the latest vaccines achieved through this recombinant technology, but not using the vaccinia virus, was obtained by our group against the myxomatosis virus and viral hemorrhagic disease of rabbits. The myxomatosis virus was used as a vector into which was inserted the gene of the VP 60 protein of the hemorrhagic virus. The result was a vaccine which induces protection against both diseases. |
|
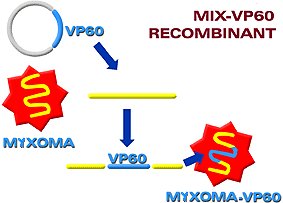
Diagram of recombinant construction between the myxomatosis virus and the vp 60 virus protein of viral hemorrhagic disease of rabbits.
|
In porcine species, a recombinant vaccine has been used experimentally, using the Aujeszky’s disease virus. The gE gene is deleted and the gp 55 glycoprotein gene of the classical swine fever virus is inserted in order to induce immunity against both diseases. The advantage of this vector is its species-specificity, but the disadvantage is that we do not always wish to use a live vaccine against Aujeszky’s disease. Other approaches being studied use specific porcine viruses, such as non-pathogenic porcine poxviruses. These have the great advantage of a capacity for the insertion and expression of genes, host specificity and the fact that they are not linked to any disease. Finally, the possibility of using the adenovirus as a vector is also being studied experimentally, given that it is not pathogenic, but it replicates and expresses many cell systems.
|
|
Electron microscopy of viral particles of myxoma and viral hemorrhagic disease of rabbits. |
These induce a great humoral and cell immune response, even at the level of the mucosa. With regard to bacterial vectors, the most used up to now have been an attenuated variant of Salmonella which induces a good immune response, even at the level of the enteric mucosa. However, it could present diagnostic problems in porcine species.
|
|
DNA VACCINES |
| Lately, the possibility of directly using a fraction of purified DNA that contains the gene of the protein capable of inducing a protective immune response has been studied. This DNA fraction is inserted into a plasmid, which serves as a vector. Animal cells capture these plasmids by processes which are still not well understood, and incorporate them into the cell nucleus, permitting the expression of the foreign gene and the production of the corresponding protein. This protein is expressed on the cell surface or is released into the medium, meaning that the immune system can recognise it in its native form as it would during natural infection with the complete agent, therefore inducing an excellent immune response. |
|
| |

